Latent Heat Of Fusion Of Ice Experiment Discussion
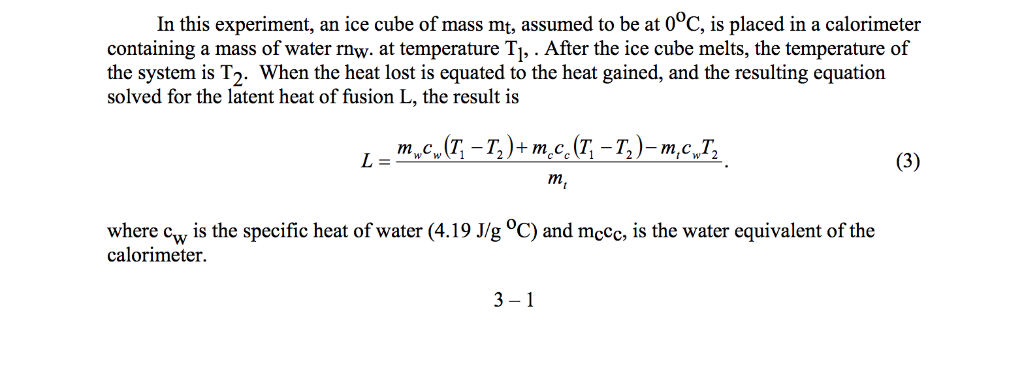
Heat lost by water is equal to the mass times the specific latent heat of fusion and the equation is vti ml m is mass of water in difference t stands for the time taken for heat the ice and l is the latent heat of fusion of ice.
Latent heat of fusion of ice experiment discussion. In objective 3 of this experiment you are to determine the latent heat of fusion of ice. Changing from a liquid to a gas then l is known as the latent heat of vaporization and is written an lv. It warms the water formed by the melting ice from zero to the final temperature. Published on may 4 2016 in this video we shall talk about an experiment to measure the specific latent heat of fusion of ice.
The amount of water and its temperature will be measured before adding some ice and then again after the ice has been melted. And these are specific to the different states of water. It melts the ice. Here you will add some ice cubes to a water bath at room temperature.
It should be noted that an object must first reach a critical temperature before it will begin melting or vaporizing i e. If the object is being vaporized i e. We shall introduce the experimental setup and mention the precautions. The actual latent heat of ice as mentioned in the introduction is 333 joules gram.
This means that the result obtained from this experiment was 11 6 inaccurate or 100 11 6 88 4 88 4 accurate. Collaborate visually with prezi video and microsoft teams. The needed energy will come from a cup of warm water. How to make an impression in a remote setting.
Instructions set up the apparatus as shown in the diagram but. The latent heat of fusion measured in cal g to fuse means to melt. Ice will be added to a calorimeter containing warm water. Thereforel 2700j l 2 7 105jkg 1 and it is not very similar to the exact value of the latent heat of fusion of ice which is 3 3 105 jkg 1.
The latent heat of fusion of ice this experiment is designed to measure the amount of heat needed to turn 1 kg of ice at 0 o c into 1 kg of water at 0 o c you will need an electric immersion heater a funnel a glass beaker an ammeter a voltmeter a power supply a stop clock a retort stand boss and clamp ice and a balance. And this is the amount of heat that s required to fuse 100 degree water into 100 degree ice. Heat is removed from the water bath to both melt the ice and warm the melted ice water to an equilibrium temperature. Error percentage 333 294 5 38 5 38 5 333 x 100 11 6 therefore the error was 11 6.
The heat energy lost by the water and calorimeter does two things. But this first number right here is the heat of fusion. Heat lost heat gained. If you looked up any other element or molecule you would have different values for these numbers we re going to be dealing with right now.