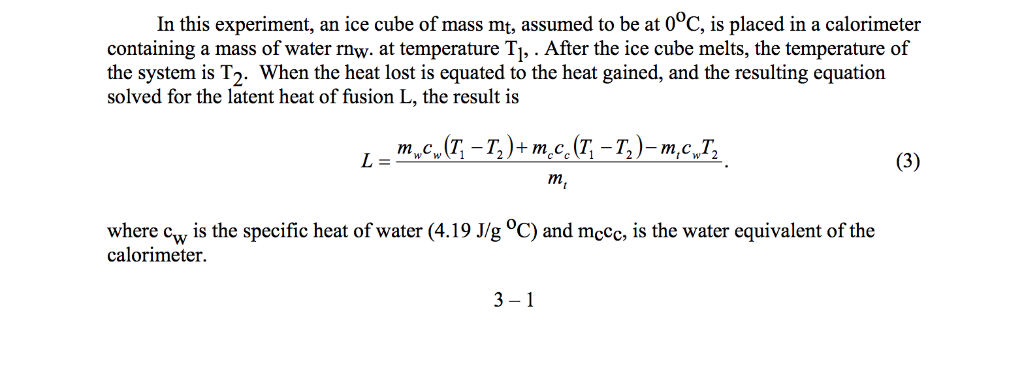Latent Heat Of Fusion Of Water Experiment
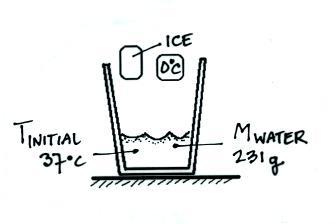
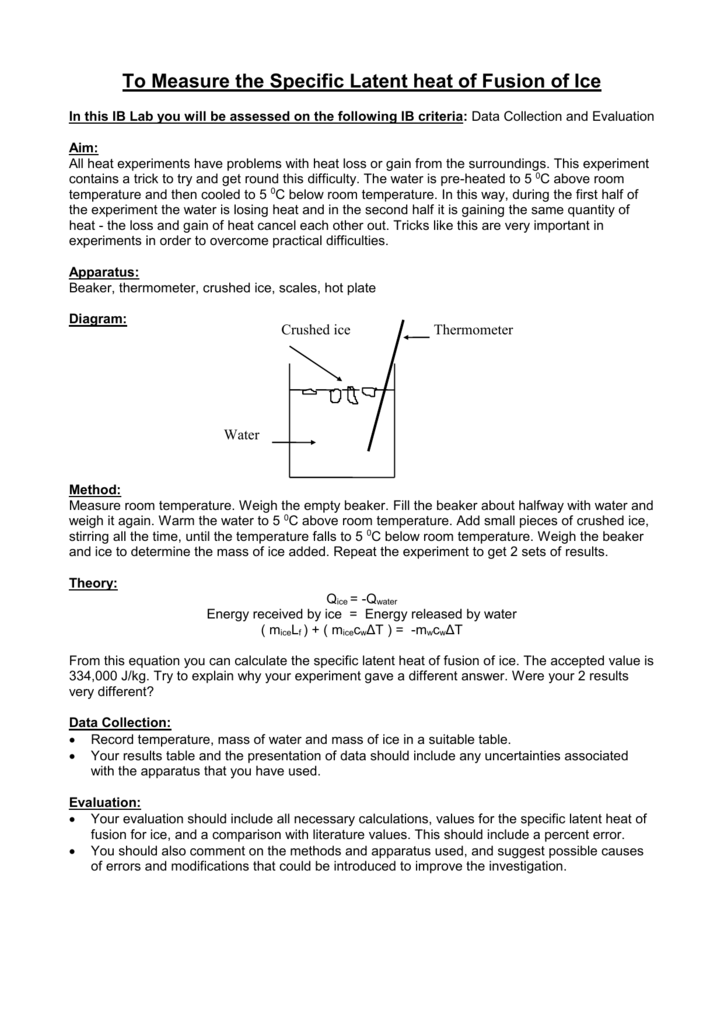
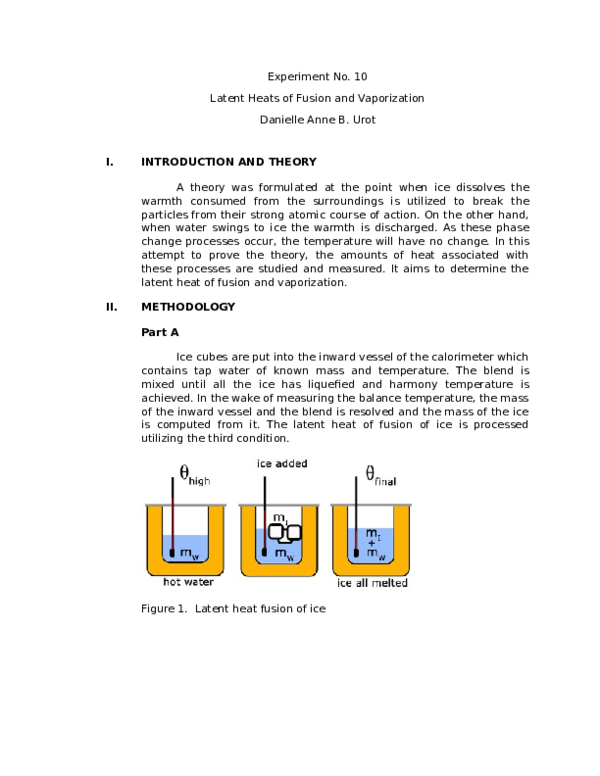
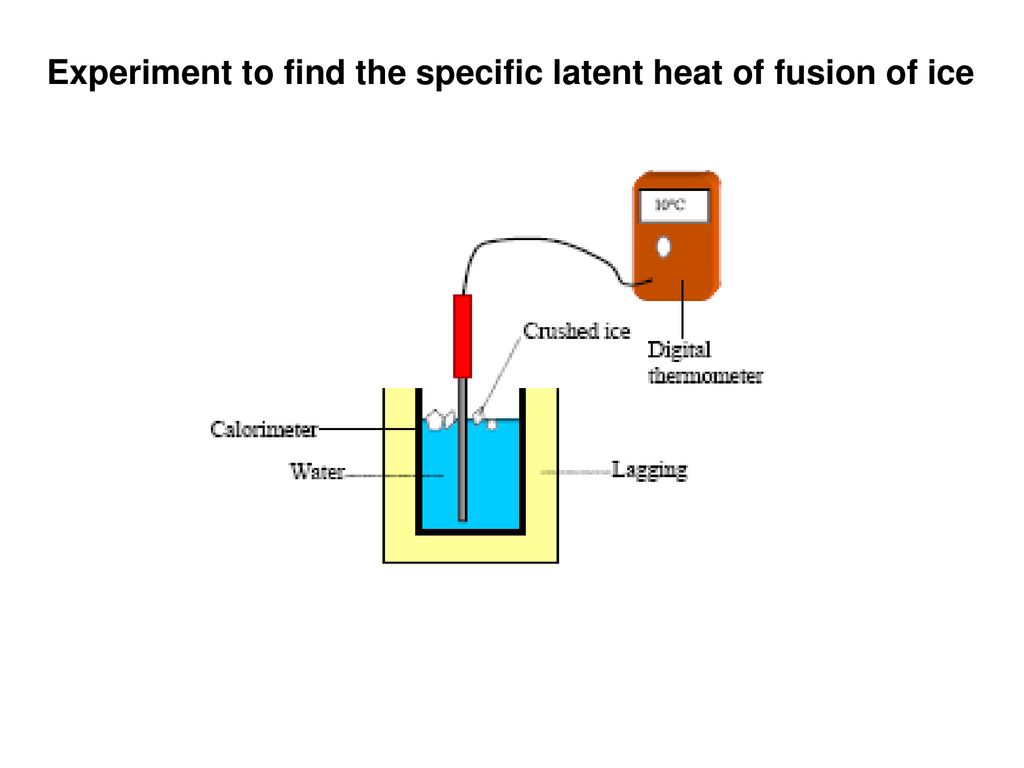
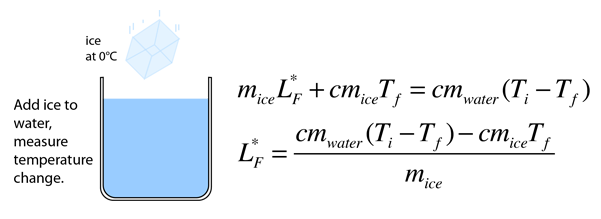
Ice will be added to a calorimeter containing warm water.
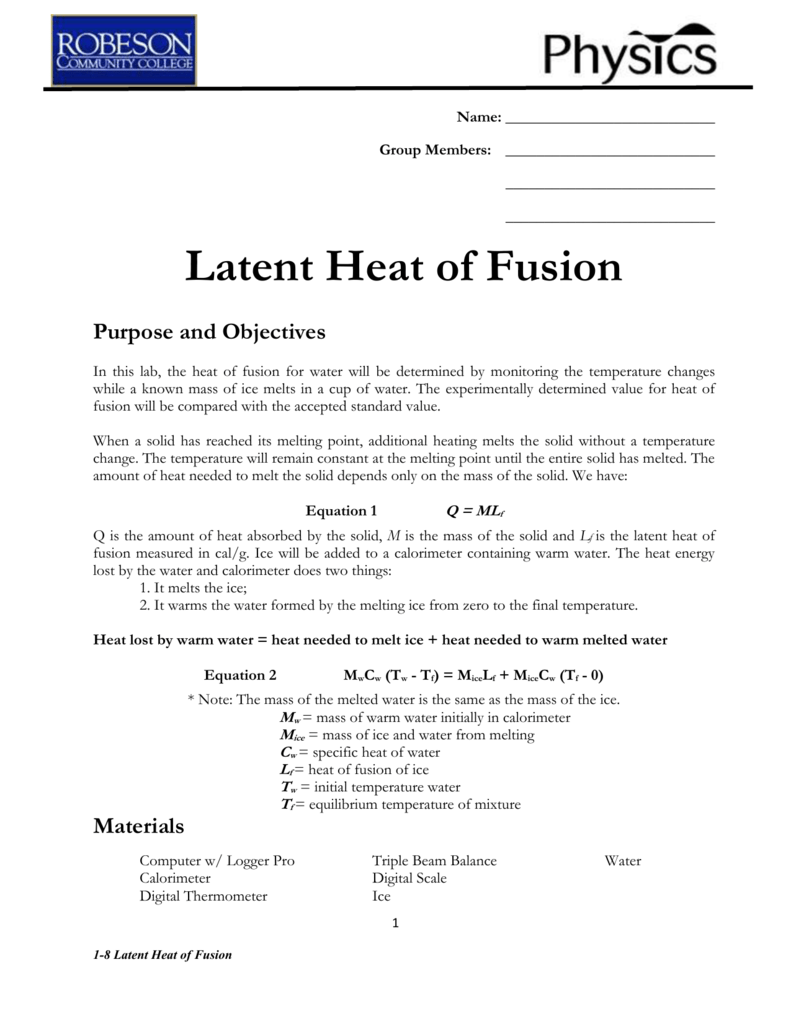
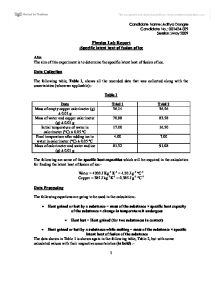
Latent heat of fusion of water experiment. The needed energy will come from a cup of warm water. The experimentally determined value for heat of fusion will be compared with the accepted standard value. Assume that the energy lost by the warm water is gained by the ice but notice that the energy is used first to melt the ice then to raise the temperature of the melted ice to match the water the final temperature of water melt ice is the same. It should be noted that an object must first reach a critical temperature before it will begin melting or vaporizing i e.
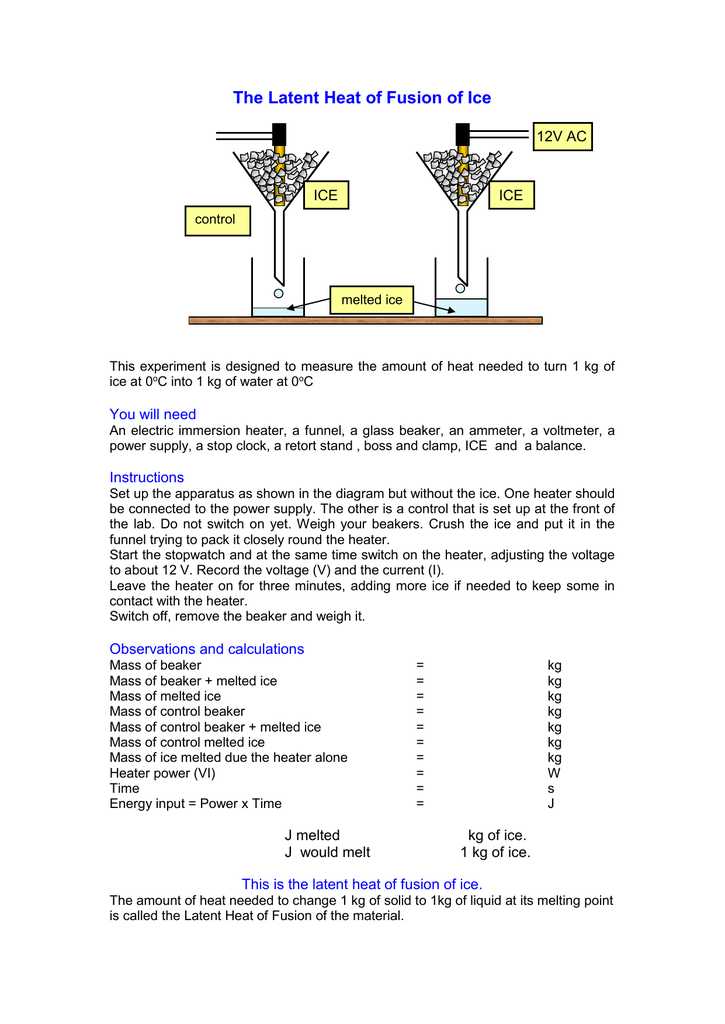
Changing from a liquid to a gas then l is known as the latent heat of vaporization and is written an lv. Experiment 4 heat of fusion and melting ice experiment in this lab the heat of fusion for water will be determined by monitoring the temperature changes while a known mass of ice melts in a cup of water. Calculate the latent heat of fusion lfor each of the three trials you performed. If the object is being vaporized i e.
In this experiment you will try to measure the latent heat of fusion of ice lhice the energy needed per gram to melt ice. The amount of water and its temperature will be measured before adding some ice and then again after the ice has been melted. The latent heat of fusion and is written as l f. Here you will add some ice cubes to a water bath at room temperature.
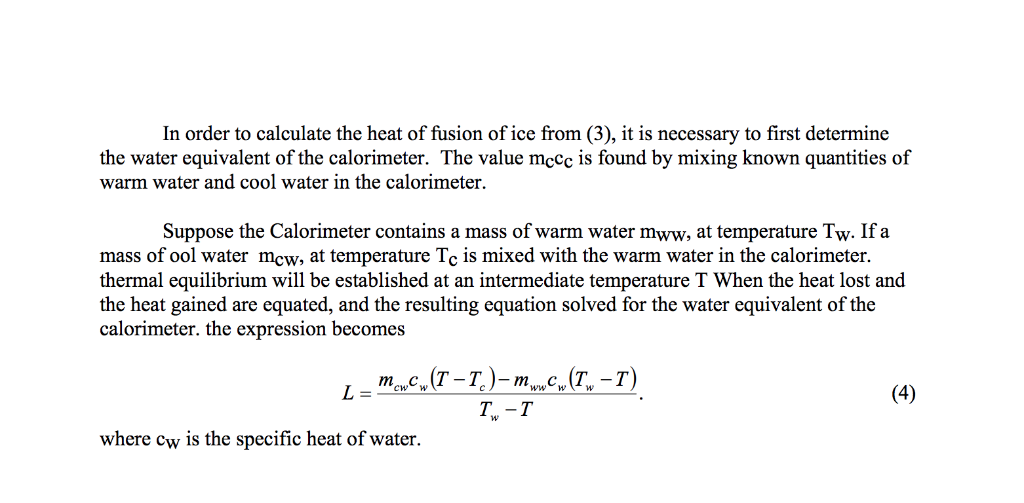
In objective 3 of this experiment you are to determine the latent heat of fusion of ice.