Latent Heat Of Fusion
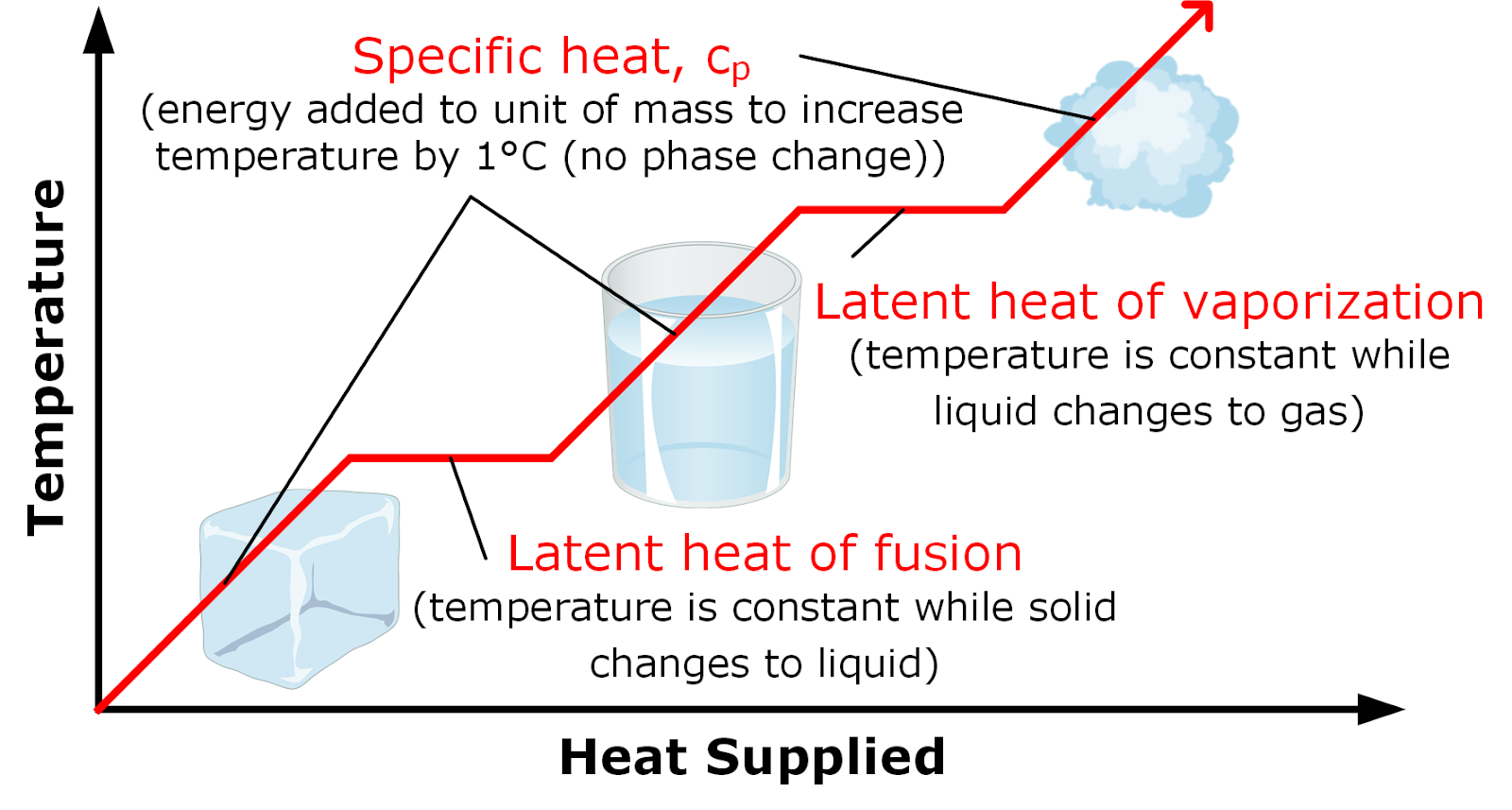
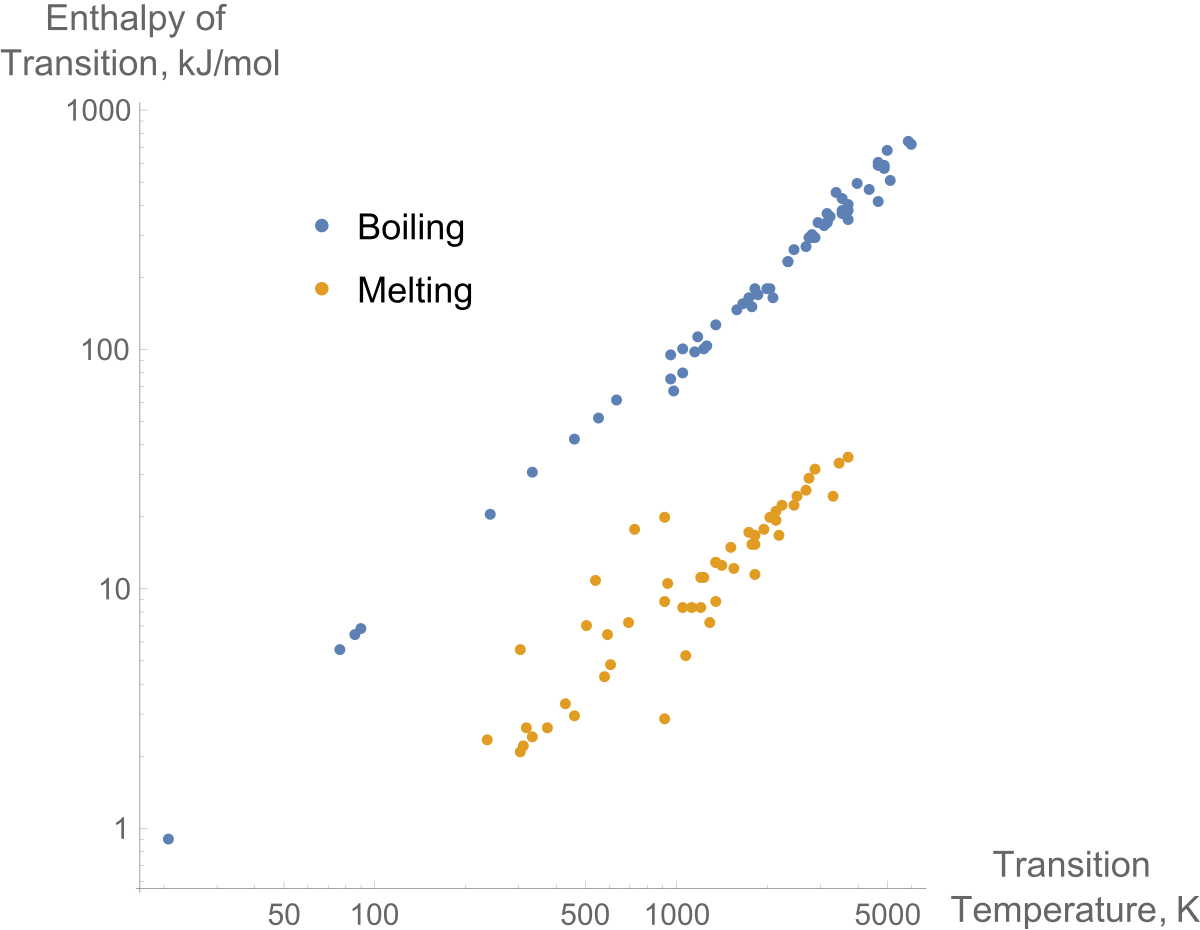
When the heat of fusion is referenced to a unit of mass it is usually called the specific heat of fusion while the molar heat of fusion refers to the enthalpy change per amount of substance in moles.
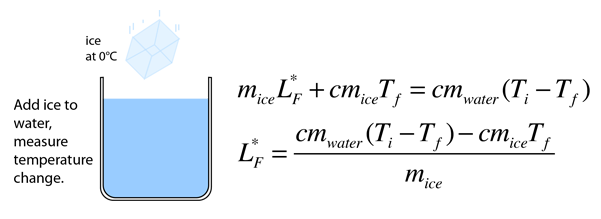
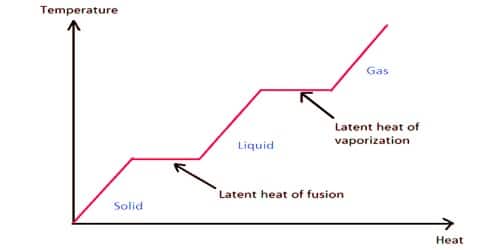
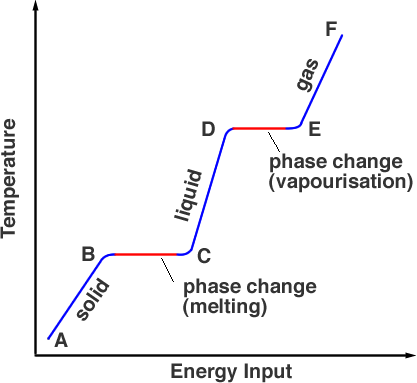
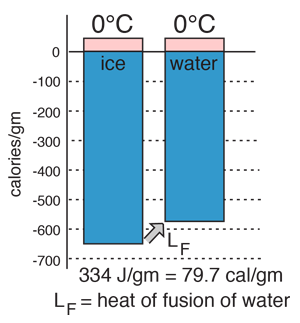
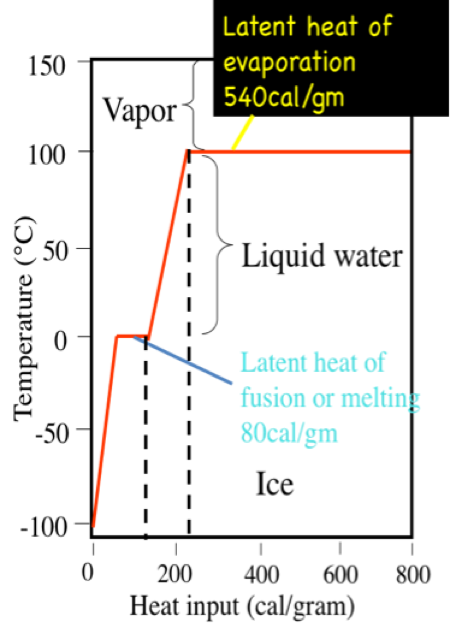
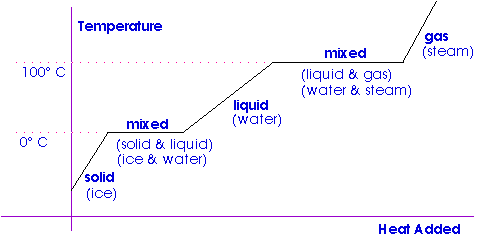
Latent heat of fusion. Then the heat absorbed by it means the latent heat of fusion formula will be q m times l f. Therefore the latent heat of fusion of a substance can be defined as the change in the enthalpy of a substance when it undergoes a phase transition from the solid. Examples are latent heat of fusion and latent heat of vaporization involved in phase changes i e. The latent heat of fusion is the enthalpy change of any amount of substance when it melts.
The formula for latent heat of fusion. A substance condensing or vaporizing at a specified temperature and pressure. 1 2 the term was introduced around 1762 by british chemist joseph black. Enthalpy of fusion is considered synonymous with latent heat of fusion because the melting of a solid under normal atmospheric pressure usually requires energy in the form of heat.